pharmacy
Canada is tightening its drug compounding regulations
Compounding mistakes have led to deaths at home and abroad this decade

caption
Maria Tasiopoulos working the pharmacy counter at Lawaton's drugstore at Robie and Spring Garden
caption
Maria Tasiopoulos working the pharmacy counter at Lawtons drugstore at Robie and Spring GardenThe National Association of Pharmacy Regulatory Authorities is in the process of updating the regulations of a relatively obscure but important practice in the pharmaceutical world: compounding.
Compounding is when a pharmacist creates a compound when the medication a patient needs isn’t commercially available, so they customize it. Certain pharmacies specialize in compounding, while others might only do it rarely.
Sabrina McLean works at a compounding pharmacy, MacKay’s PharmaChoice in Dartmouth. She is a pharmacist and compounding consultant.
“It may be because they’re allergic to a component,” she said about why someone might need a compound. “Or sometimes it’s for somebody who needs something in a form that’s not available,” Related stories
For example, if a certain tablet has lactose but a client can’t tolerate it, then McLean would order the raw chemical and create a tablet that the client could ingest.
The rollout of the new regulations is taking place in three phases. The first for hazardous sterile preparations came out in September, and the second for non-hazardous sterile preparations was released in November. The third part of the new regulations, set to come out sometime this year, will pertain to non-sterile compounding, which is the kind McLean does.
“Sterile is when you have to guarantee that you have no bacteria or viruses or funguses,” she said. “So sterile is generally when you’re making eye drops or any kind of injections, IV, something that’s going to be delivered directly into the bloodstream.”
Non-sterile is usually topical or oral.
Before these regulations, Canada never had its own standards for compounding. Pharmacists in Canada would follow the USP 797, from the U.S. Pharmacopeial Convention; the previous Canadian national guidelines to pharmacy compounding were published in 2006 and totaled 12 pages, including the title page and table of contents. The first two phases of the new Canadian regulations add up to 200 pages, and that’s without the upcoming non-sterile regulations.
Maria Tasiopoulos, a staff pharmacist at the Lawtons on the corner of Spring Garden Road and Robie Street, said her pharmacy doesn’t specialize in compounding. But, they still do it sometimes. One of the more common compounds they make is for diclofenac, an anti-inflammatory.
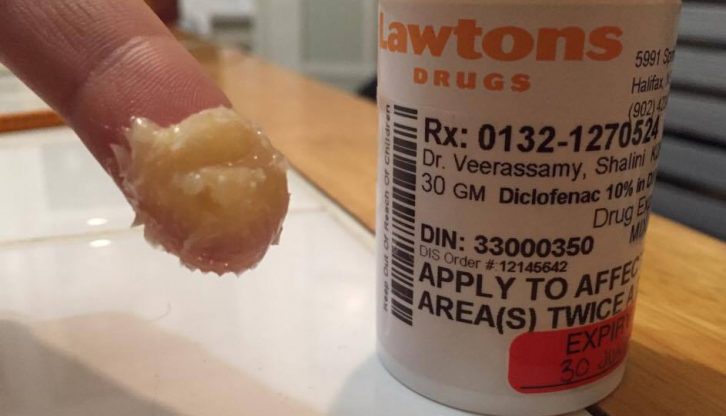
caption
A bottle of compounded diclofenac from Lawtons. The pharmacist colloquially referred to it as a ‘crointment,’ a cross between a cream and an ointment.“It’s available as a tablet, but also they’ve found a higher concentration in a cream,” she said. “You could apply it topically to a joint and you wouldn’t have the side effects of taking something internally.”
In the last few years, the amount of compounding that her pharmacy is allowed to do has decreased dramatically because of the new rules, which are meant to protect both pharmacists and their clients.
“Erectile dysfunction, that was one of the ones we did a lot of,” she said. “A lot of topical creams and stuff.”
Now, there are more compounding pharmacies that meet regulations and are equipped with the proper equipment, such as a flow hood, which is an enclosed area to prevent contamination, and capsule machine, which automatically creates capsules of medicine.
McLean said the regulations haven’t really affected her store yet, since she doesn’t work in non-sterile compounding. Even so, she understands why other pharmacies have been priced out of compounding almost entirely.
“They would have to do full renovations to meet the new requirements,” she said, which would include adding expensive equipment like the flow hood and capsule machines that Tasiopoulos’s pharmacy lacks.
Those costs haven’t yet applied to her non-sterile pharmacy, but other costs brought on by the new regulations soon will.
“We’ll have to use some more expensive personal protective equipment, more than we’re using now,” said McLean.
For example, MacKay’s will have to update its flow hood so it’s externally ventilated.
Lethal compounding errors
The new wave of regulations comes on the heels of an outbreak of fungal meningitis in the United States in 2012 that made more than 700 people sick and killed more than 60. The Nova Scotia College of Pharmacists, the provincial body that decides how to enact federal regulations, mentions the outbreak on its policies positions guidelines web page.
The epidemic was traced to the New England Compounding Center. On Wednesday, former head pharmacist and co-owner Barry J. Cadden was found guilty of fraud and racketeering in relation to the outbreak.
In Canada, compounding errors have had fatal results here as well. In October, a young boy in Ontario died when a compounding pharmacy accidentally swapped his sleeping pill with a powerful relaxant.
McLean has an idea of how that might have happened, although she doesn’t know for sure.
“Maybe they were making two different things at the same time so they had several chemicals out on the counter because they were working on two different compounds, and maybe they got switched,” she said.
Many of the ingredients come from the same company, which means their packaging looks the same. McLean has decided to only ever work on one compound at a time, and every compound must be double-checked by another staff member before it goes out.

